Relation between Nernst equation and the pH meter

According to Nernst Equation, in a chemical reaction
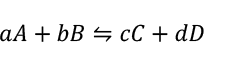
The potential of this reaction is given by the equation
![𝐸=𝐸0−𝑅𝑇/𝑛𝐹 𝑙𝑛 [𝐶]𝑐[𝐷]𝑑/[𝐴]𝑎[B]𝑏](../images/能斯特方程式截圖/002.png)
Diagram of the glass electrode can be represented as:
![𝑹𝒆𝒇𝟏‖[𝐇+]┤| Glass membrane|[𝐇+]|𝑹𝒆𝒇𝟐](../images/能斯特方程式截圖/003.png)
The H+ ion exchange with the glass membrane to produce the inner and outer potential E1 & E2
![H+Glass1- ⇋ H+1 + Glass1- H+1 : 外部溶液氫離子濃度
𝐸1=𝐸10−0.0592/1 log〖[𝐻+]1/([H+]𝐺𝑙𝑎𝑠𝑠1)〗H+Glass2- ⇋ H+2 + Glass2- H+2 : 內部溶液氫離子濃度
𝐸2=𝐸20−0.0592/1 log〖[𝐻+]2/([H+]𝐺𝑙𝑎𝑠𝑠2)〗](../images/能斯特方程式截圖/004.png)
By measuring the boundary potential (Eb) the relation between Eb and pH is:
![𝐸𝑏=𝐸1−𝐸2 (Eb : Boundary Potential)
𝐸𝑏= (𝐸10−0.0592/1 log〖[𝐻+]1/([H+]𝐺𝑙𝑎𝑠𝑠1) 〗)− (𝐸20−0.0592/1 log〖[𝐻+]2/([H+]𝐺𝑙𝑎𝑠𝑠2) 〗)
=𝐸10−0.0592 log[𝐻+] 1+0.0592 log[𝐻+]𝐺𝑙𝑎𝑠𝑠1−(𝐸20−0.0592 log[𝐻+]2+0.0592log[H+]𝐺𝑙𝑎𝑠𝑠2)
=(𝐸10−𝐸20+0.0592 log[𝐻+] 𝐺𝑙𝑎𝑠𝑠1−0.0592 log[𝐻+] 𝐺𝑙𝑎𝑠𝑠2+0.0592 log[𝐻+] 2)−0.0592 log[𝐻+] 1
=(𝐸10−𝐸20+0.0592log ([H+]𝐺𝑙𝑎𝑠𝑠1)/([H+]𝐺𝑙𝑎𝑠𝑠2)+0.0592log[𝐻+]2)−0.0592 log[𝐻+] 1](../images/能斯特方程式截圖/005.png)
Which E10, E20 are the formal potential, the [H+]Glass is constant and the inner proton ion concentration [H+]2 is invariable as constant. The equation can be expressed as:
![𝐸𝑏=𝐸𝑘′−0.0592log[H+]1
𝐸𝑏=𝐸𝑘′+0.0592p𝐻](../images/能斯特方程式截圖/006.png)
Due to the proton ion at the glass membrane and the inner proton ion concentration will change after measuring, the 𝐸𝑘′ will be changed. So the glass electrode needs to be calibrated before use.

According to Nernst Equation, in a chemical reaction
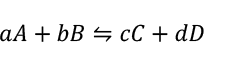
The potential of this reaction is given by the equation
![𝐸=𝐸0−𝑅𝑇/𝑛𝐹 𝑙𝑛 [𝐶]𝑐[𝐷]𝑑/[𝐴]𝑎[B]𝑏](../images/能斯特方程式截圖/007.png)
Humming Probe strip electrode modify the proton ion selective membrane at the electrode surface which having the redox reaction with proton ion, the diagram can be represented as:
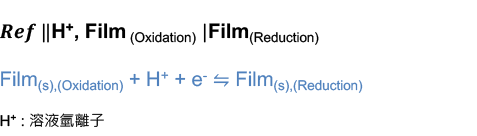
According to Nernst Equation, the electrode potential is
![𝐸=𝐸0−0.0592/1 log〖1/([𝐻+])〗
𝐸=𝐸0+0.0592log[𝐻+]
𝐸=𝐸0−0.0592p𝐻](../images/能斯特方程式截圖/009.png)
Due to the precision and the consistency of the strip electrode, the 𝐸0 are the same for every electrode. And the strip electrode need not to be calibrated.
Think it's a good article?
Share it with others

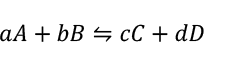
![𝐸=𝐸0−𝑅𝑇/𝑛𝐹 𝑙𝑛 [𝐶]𝑐[𝐷]𝑑/[𝐴]𝑎[B]𝑏](../images/能斯特方程式截圖/002.png)
![𝑹𝒆𝒇𝟏‖[𝐇+]┤| Glass membrane|[𝐇+]|𝑹𝒆𝒇𝟐](../images/能斯特方程式截圖/003.png)
![H+Glass1- ⇋ H+1 + Glass1- H+1 : 外部溶液氫離子濃度
𝐸1=𝐸10−0.0592/1 log〖[𝐻+]1/([H+]𝐺𝑙𝑎𝑠𝑠1)〗H+Glass2- ⇋ H+2 + Glass2- H+2 : 內部溶液氫離子濃度
𝐸2=𝐸20−0.0592/1 log〖[𝐻+]2/([H+]𝐺𝑙𝑎𝑠𝑠2)〗](../images/能斯特方程式截圖/004.png)
![𝐸𝑏=𝐸1−𝐸2 (Eb : Boundary Potential)
𝐸𝑏= (𝐸10−0.0592/1 log〖[𝐻+]1/([H+]𝐺𝑙𝑎𝑠𝑠1) 〗)− (𝐸20−0.0592/1 log〖[𝐻+]2/([H+]𝐺𝑙𝑎𝑠𝑠2) 〗)
=𝐸10−0.0592 log[𝐻+] 1+0.0592 log[𝐻+]𝐺𝑙𝑎𝑠𝑠1−(𝐸20−0.0592 log[𝐻+]2+0.0592log[H+]𝐺𝑙𝑎𝑠𝑠2)
=(𝐸10−𝐸20+0.0592 log[𝐻+] 𝐺𝑙𝑎𝑠𝑠1−0.0592 log[𝐻+] 𝐺𝑙𝑎𝑠𝑠2+0.0592 log[𝐻+] 2)−0.0592 log[𝐻+] 1
=(𝐸10−𝐸20+0.0592log ([H+]𝐺𝑙𝑎𝑠𝑠1)/([H+]𝐺𝑙𝑎𝑠𝑠2)+0.0592log[𝐻+]2)−0.0592 log[𝐻+] 1](../images/能斯特方程式截圖/005.png)
![𝐸𝑏=𝐸𝑘′−0.0592log[H+]1
𝐸𝑏=𝐸𝑘′+0.0592p𝐻](../images/能斯特方程式截圖/006.png)

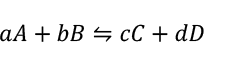
![𝐸=𝐸0−𝑅𝑇/𝑛𝐹 𝑙𝑛 [𝐶]𝑐[𝐷]𝑑/[𝐴]𝑎[B]𝑏](../images/能斯特方程式截圖/007.png)
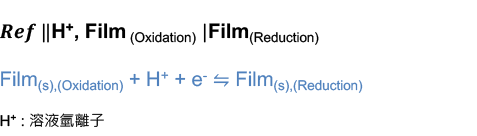
![𝐸=𝐸0−0.0592/1 log〖1/([𝐻+])〗
𝐸=𝐸0+0.0592log[𝐻+]
𝐸=𝐸0−0.0592p𝐻](../images/能斯特方程式截圖/009.png)
